Keeping accurate records not only is best practice for good data management, it is an important component for maintaining compliance with federal regulations, institutional requirements, and professional standards. iProtocol retains every protocol version created and submitted; each protocol version retains the versions of any uploaded documents including consent forms, permission forms, and study instruments. iProtocol helps researchers retain the documents they need in order to document compliance with IRB-SBS review (though researchers will need to retain consent documents, etc., used during the study as well; please see Record Keeping for more information).
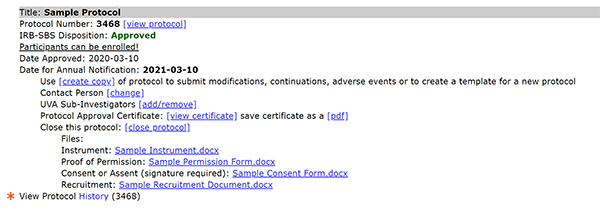
To access a protocol's history:
- Select View Protocol History (link below approved protocol).
- Protocol History page opens in a separate tab.
