Studies may be chosen for a Post Approval Monitoring (PAM) review for any of the following reasons:
- At random (studies are selected on a monthly basis)
- At the request of the IRB-SBS staff, IRB Chair, study team member as an educational tool, or where compliance concerns have been raised
- As a result of complaint by a research participant which raises safety or compliance concerns
- When continuing review suggest that changes may have occurred without IRB approval
- When a protocol involves vulnerable populations, or unusual levels or types of risks to subjects
Once a protocol has been selected for a PAM review an automated email is sent to the Principal Investigator (PI) with instructions to complete a compliance self-assessment within a set period of time.
If the PI is not available following the PAM notification due to travel or other circumstances, please inform the post approval compliance monitor of the situation and a date that is mutually convenient can be arranged for the visit.
If there is no response to the initial PAM notification, the PI will receive a follow up email from the compliance monitor, and if applicable, the following individuals listed on the protocol in iProtocol will be copied: Faculty Advisor, Faculty Sponsor, or Contact Person.
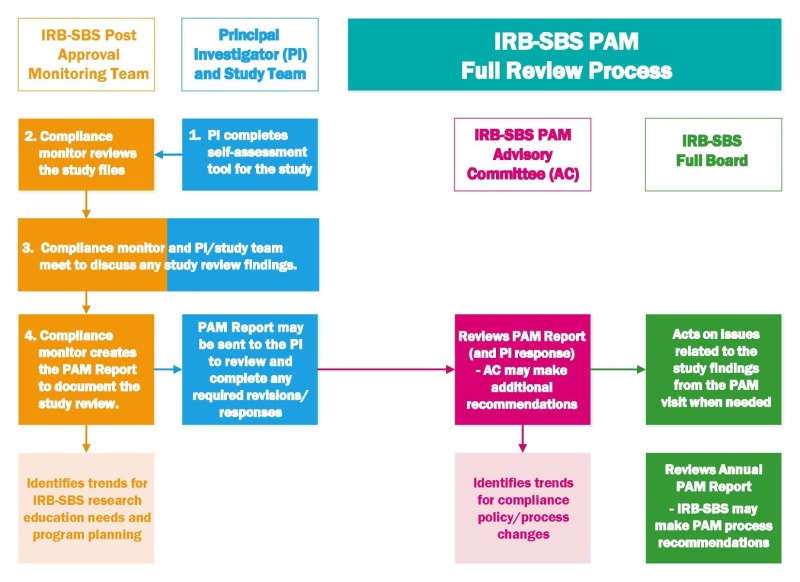
The post-approval monitoring process for a full study review has 4 steps:
- The investigator will complete a compliance self-review via the investigator self-assessment tool.
- The compliance monitor will complete a review of the study documents (IRB approved protocol and documents, participant records, consent documents, etc.) via remote or on-site methods.
- The investigator and the compliance monitor will meet (remote or in-person) to discuss the review findings and any recommended follow up actions.
- The compliance monitor will write the PAM report to document the review, issues identified, and corrective and preventative action plans.
During the PAM visit, the compliance monitor may review any of the following:
- Correspondence with the IRB and any other applicable institutional oversight committees
- Regulatory documents
- Original signed and dated consent forms for each subject or a sample of subjects
- Research records (screening log, subject enrollment log, procedure documentation)
- Written documentation of the consent process for each subject
- Study subject records and any source documents (such as written surveys, videotapes , field notes)
- Serious and/or unexpected adverse event documentation and copies of required reports to the IRB, and other appropriate entities
- Data entered for analysis
Please recognize that the amount of time needed for a review varies greatly from study to study. The time needed to review the study documents depends on the complexity of the study, the type of review, the number of subjects, and any issues identified.
The post-approval compliance monitor recognizes that your time as an investigator is valuable. Cooperation is key to the PAM visit and as such, the compliance monitor will make an effort to work with your schedule while not sacrificing the quality of the review.
When the monitoring visit is complete, the compliance monitor will:
- Meet briefly with the PI (and study team members) to discuss any findings (step 3 of PAM review)
- Write a PAM Report of the findings (step 4 of PAM review)
- If requested, the compliance monitor may send a draft copy of the PAM Report to the PI for review and comment.
- The PAM Report for the study will be reviewed by the PAM Advisory Committee to discuss finding and to be assigned a compliance rating. The PAM Advisory Committee generally meets on a monthly basis.
- The PI will receive a PAM Letter from the PAM Advisory Committee with details for any follow up actions required and to notify the PI of the studies compliance rating. Please note, studies with a compliance rating of exceptional or satisfactory (i.e. good rating) will receive the PAM Letter within a 6 month period as the letters for these studies are batched.
Often minor compliance issues can be addressed the during the review or on the day of the compliance monitor's meeting with the PI and study team. The PAM Advisory Committee and/or IRB-SBS may request that the monitor follows up with the PI and study team and/or request that the PI and study team participate in educational activities.
Contact the IRB-SBS PAM & Ed Program:
For questions or concerns about the IRB-SBS PAM & Ed program, please email: IRBResearchEducation@virginia.edu
Please subscribe to our Quarterly Investigator Newsletter for the latest Education events and IRB-SBS updates.
Stephanie Keister, MS, Ed.D.
Post Approval Monitor and Educator
IRB for the Social and Behavioral Sciences
Messenger Mail: Box 800392